Inorganic feed phosphate is a stable phosphorus (P) source in animal nutrition providing a high P quality. In contrast, the P supply from cereals is low, variable, and a large part is not digestible by animals. As mentioned in Rostagno 2017 study, not all plant materials are equal in terms of P content and percentage of phytic acid. Moreover, P can also fluctuate from one source to another and from the quality of the land it was cultivated in. Mineral P from feed phosphates is less dependent of external factors.
Most of feed phosphates are mined from phosphate rock and synthesized artificially to make the P available for animals. These phosphates provide a stable composition, low in impurities and considered by scientists as the best available source of P for animals. An accurate calculation of P levels to reach animals’ nutritional needs is important from both nutritional and environmental point of view. A lot of different feed phosphates are used on the market with various quality. A combination of simple in vitro tests helps to quickly assess the product quality in the laboratory. The quality can differ greatly in chemical composition (strongly correlated with P digestibility) and purity (undesirable element content). It is a key point for nutritionists to be aware of the products quality in order to make the best choice to optimize P supply to the animals.
- Naming of feed phosphates: Ca/P ratio
Before checking the quality, it is important to understand the naming of a feed phosphate. Typically, a MCP contains approximately 22.7 % P, a MDCP 21 % P and a DCP 18 % P. Calcium content for MCP and MDCP products ranges from 15 to 18 % while a DCP product contains around 24 % calcium. Those percentages can vary slightly depending on the quality of the phosphoric acid used and the production process. Thus, we can observe a multitude of phosphates on the market. P is not the only parameter to determine the name. The Ca:P ratio (figure 1) is a good tool to categorize and quickly identify the nature of a calcium phosphate.
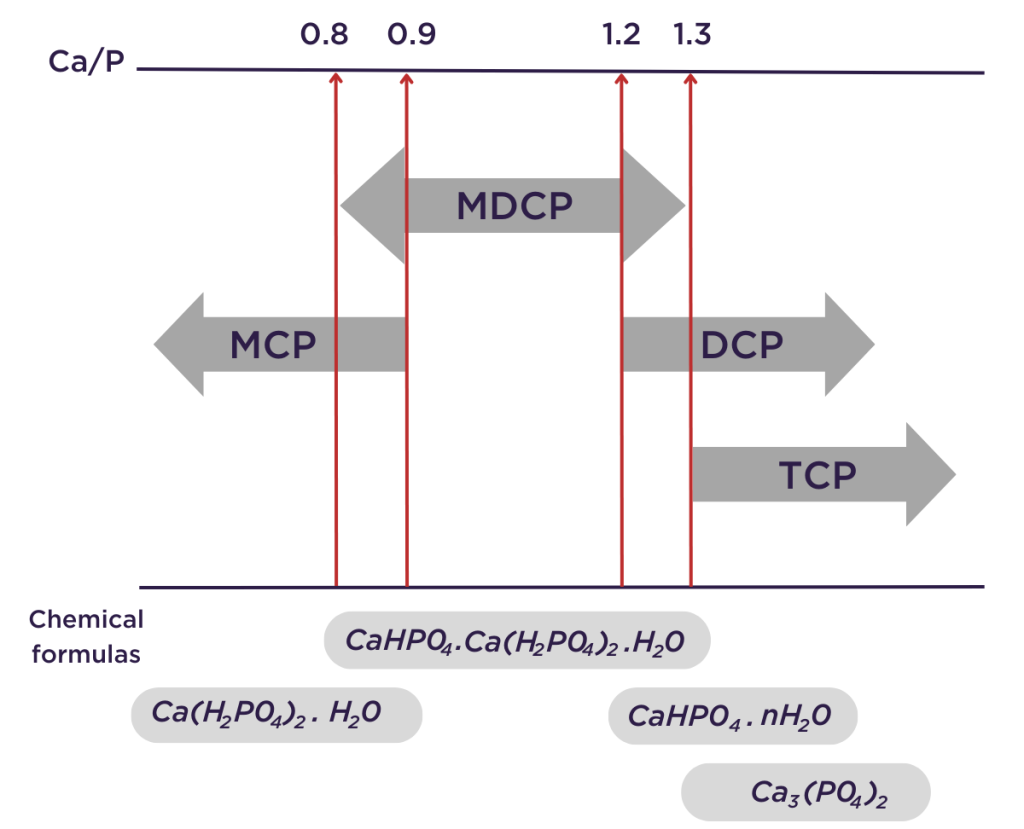
Figure 1: Ca/P ratio according to the Commission Regulation (EU) No 68/2013
on the Catalogue of feed materials
- Feed phosphate purity: undesirable substances
Elements such as heavy metals come from the rock from which phosphoric acid is produced. When feed phosphates are manufactured, de-fluorinated phosphoric acid needs to be used to ensure that phosphates are low in those undesirable elements. In the European Union, maximum levels have been set for lead, cadmium, mercury, arsenic, and fluorine (table 1) in animal feed according to the directive 2002/32/EC on undesirable substances in animal feed relative to a feedstuff with a moisture content of 12 %. It is important to consider those maximum levels of undesirable substances in order to maintain a healthy animal physiology.

Table 1: Maximum of undesirable substances in terms of inorganic contaminants in feed phosphates, expressed in mg/kg (ppm)
- In vitro P solubilities: a practical tool to check phosphates quality
It is important to understand that commercial feed phosphates are not pure products. A calcium phosphate is composed of 3 types of molecules: MCP, DCP and TCP. Thus, it can exist under different chemical forms depending on the concentration of each of these molecules within the phosphate.
As explained in the “Guidance of identification and naming of substances under REACH and CLP” of ECHA, “a mono-constituent substance is a substance in which one constituent is present at a concentration of at least 80% (w/w) and which contains up to 20% (w/w) of impurities”. It also indicates that “a mono-constituent substance is named according to the one main constituent”. For example, in REACH registration of MCP, MCP is considered as a mono-constituent because MCP molecule (Ca(H2PO4)2.H2O) is over 80%. Moreover, it considers DCP molecule (CaHPO4) as an impurity with a maximal concentration of 20% (w/w).
Although in vivo tests are essential for accurately determine a phosphate’s digestibility or availability, they cannot be carried out on a routine basis. It is therefore recommended to carry out quick in vitro analysis to validate the phosphate quality used in your plants.
Cauduro (2009) worked on several in vitro test to find the best suited one. 8 in vitro methods were tested to analyze 6 P sources whose digestibility in pigs was determined by in vivo tests. Correlations between in vitro and in vivo methods are shown in Table 2. The most suitable test for rapid phosphate screening is the 2% citric acid solubility test, which Phosphea uses routinely. These results confirm the value of this method, developed in the 1960s by M. Gueguen of INRAE, in ruminants (figure 2). This test provides an indication of the level of P availability.
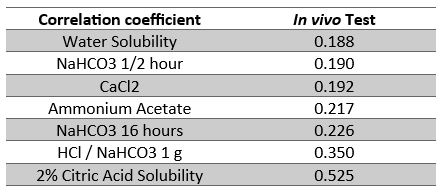
Table 2 : Correlation between in vivo P digestibility and in vitro analysis methods. Cauduro, J 2009, In vitro testing of inorganic P sources for P availability in swine, Master Thesis, Applied Sciences, RMIT University

Figure 2: Correlation between citric acid solubility, alkaline ammonium
citrate and true absorption coefficient (Guéguen, 1975)
On the other hand, the P solubility in alkaline ammonium citrate points to the chemical nature of the product and shows the presence of TCP molecules. P that is more than 90% soluble in both test is of high nutritional quality (Guéguen, 1970). The relationship between the true absorption coefficient (TAC) of different phosphates and their solubility in citric acid and ammonium citrate has been determined by INRAE (Figure 2). From these results, it can be observed that a phosphate’s TAC increases linearly in relation to its solubility in both media.
P water solubility give an information about the molecular composition of phosphates. Indeed, P water solubility is positively correlated to the MCP molecule content in calcium phosphates and thus the digestibility of feed phosphates (NRC, 2011). The higher the solubility is, the higher the digestibility is (Figure 4). In Figure 3, we can see that P will not be absorbed in the same way depending on the form in which it is found. In vivo tests show that MCP and DCP molecules are better absorbed by monogastric animals than the TCP form which is less soluble (Cromwell 1989, Guéguen 1988).
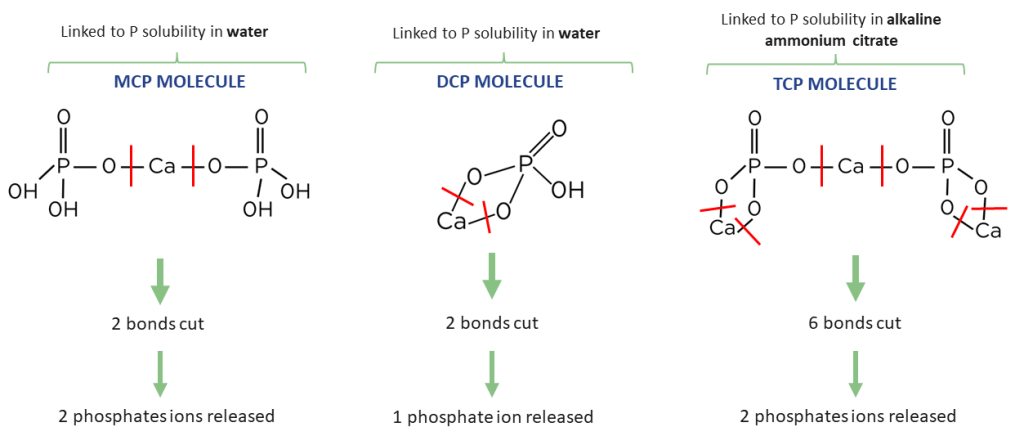
Figure 3: MCP, DCP and TCP molecules availability
Figure 4 presents results of trials carried out by Phosphea. We can easily see the correlation between the P water solubility and the P digestibility:
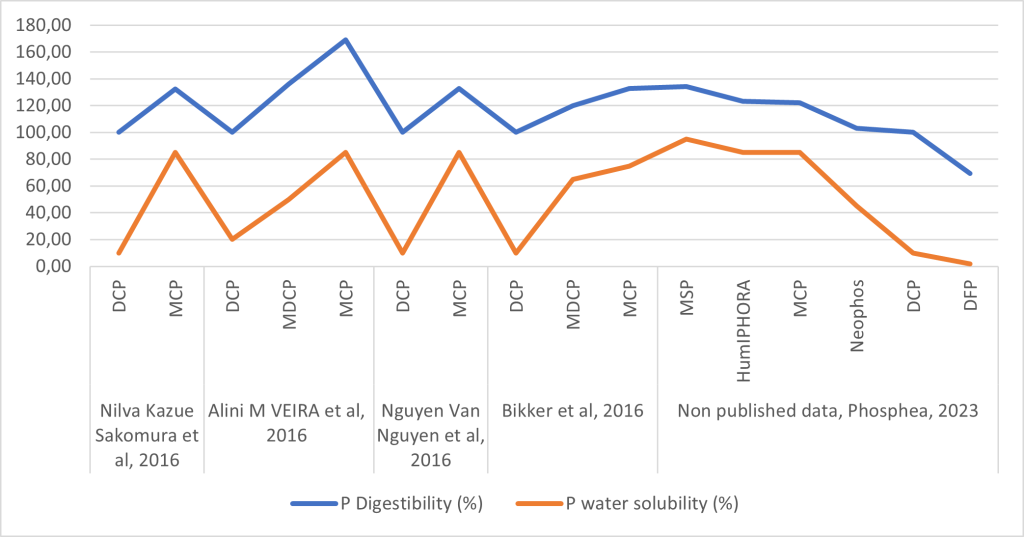
Figure 4: Correlation between P water solubility and P digestibility with DCP as base 100 (based on Bikker et al, 2016 ; Nilva Kazue Sakomura et al, 2016 ; Alini M VEIRA et al, 2016 ; Nguyen Van Nguyen et al, 2016 ; non-published data, Phosphea, 2023)
- CEFIC
Phosphea is a member of the European Chemical Industry Council (CEFIC) and active in the IFP (Inorganic Feed Phosphate) Group. Regarding phosphates quality, the CEFIC recommend that both, solubilities of P in citric acid and alkaline ammonium citrate values should be above 95% and that phosphates comply with European regulations on heavy metals.
IFP Group members participate in an annual ring test on phosphates directly sampled at the product sites by an independent company. The anonymous samples are analysed by each member’s laboratory. This ring test is also an opportunity to verify our laboratory’s accuracy regarding the following parameters: P, Ca, P citric acid solubility, alkaline ammonium citrate solubility, and heavy metals.
The European producers of inorganic feed phosphates grouped under the IFP Sector Group can display the following logo which reflects their membership of the IFP Group:

In conclusion, P is a key mineral and must be well included in animal diets. Thus, it is essential to control the quality of commercial inorganic feed phosphate sources to get the correct amount of P in animal diet. Phosphea feed phosphates are regularly analysed using standardised laboratory methods to determine P and Ca content, solubilities in citric acid and in ammonium citrate, and heavy metals content. In the next newsletter, we’ll describe the in vivo tests analysis methods that allow to accurately determine a phosphate’s digestibility or availability.
For more information, don’t hesitate to contact our technical team: technical@phosphea.com!
